Apart from adipose tissue, which is one of the major sources of adult stem cells, bone marrow also serves as an important source. One of the most widely used types of bone-marrow-derived stem cells in therapy is Bone Marrow Mononuclear Cells (BM-MNC) which consist of various cell populations such as hematopoietic stem and progenitor cells (HSC/HPC) lymphoid cells, monocytes, endothelial progenitor cells (EPCs), and mesenchymal stem cells (MSCs)1. Here is the BM-MNC isolation procedure performed at ProSTEM. The processing workflow can be seen in the diagram below.
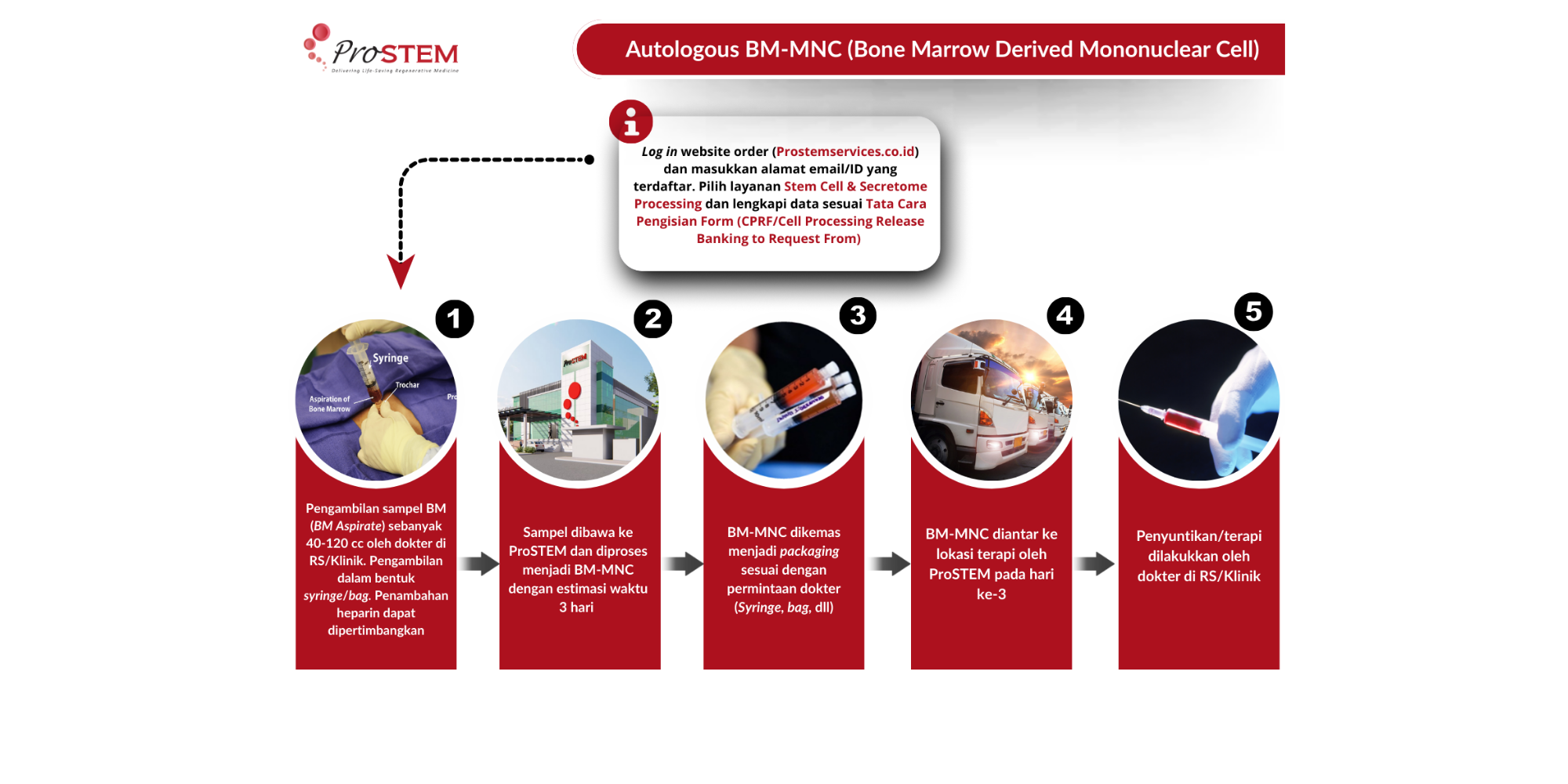
BM-MNC used for therapeutic purposes in clinical trials are typically obtained through a bone marrow aspiration procedure from the patient and subsequently reinfused into the same patient (autologous). The aspirate then undergoes an automated separation process at ProSTEM to obtain BM-MNC, which can be directly injected into the patient or stored in a frozen state for future use. The use of an automated cell-separation device enables a shorter processing time and reduces the risk of microbial contamination, as well as producing a higher recovery of mononuclear cells with preserved, or even enhanced, functional capacity.2
Autologous BM-MNC therapy harnesses a wide range of cells in a diverse mix of mesenchymal stromal cells and hematopoietic stem cells, each with distinct immunomodulatory, anti-inflammatory, and regenerative properties. The capacity of these cells to release trophic factors that support tissue repair, inhibit apoptosis, and foster neuroprotection is important for treating conditions with limited benefit from traditional treatments. Autologous BM-MNC can migrate to injury sites and exert therapeutic effects by neuroprotective and angiogenic mechanism.3
References
- Yunir E, Kurniawan F, Rezaprasga E, Wijaya IP, Suroyo I, Matondang S, Irawan C, Soewondo P. Autologous Bone-Marrow vs. Peripheral Blood Mononuclear Cells Therapy for Peripheral Artery Disease in Diabetic Patients. International Journal of Stem Cell. 2020; DOI: 10.15283/ijsc20088.
- Nagai H, Miwa A, Yoneda K, Fujisawa K, Takami T. Optimizing the Seeding Density of Human Mononuclear Cells to Improve the Purity of Highly Proliferative Mesenchymal Stem Cells. Bioengineering (Basel). 2023; DOI: 10.3390/bioengineering10010102.
- Kala S, Aggarwal A, Singh Rajput B, et al. (December 12, 2024) Safety and Efficacy of Autologous Bone Marrow Derived Mononuclear Cell Transplant in the Management of Various Neurological Disorders. Cureus 16(12): e75617. DOI 10.7759/cureus.75617

