Retinitis pigmentosa is a hereditary disease that affects the retina, especially the rod photoreceptor cells, and can lead to blindness (Hamel, 2006). This disease, commonly suffered by young individuals, is progressive and currently has no cure. Clinical symptoms of retinitis pigmentosa include a decrease in field of vision accompanied by a decline in photoreceptor function, which can ultimately lead to blindness.
Retinitis pigmentosa is a disorder with high variability. Some patients experience visual decline since childhood, while others may not show symptoms until adulthood. The symptoms of visual decline occur due to the gradual death of two types of photoreceptors: rod photoreceptors and cone photoreceptors (Hartong et al., 2006).
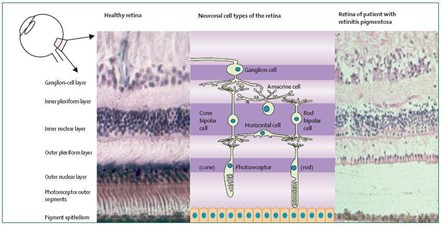
Gambar 1. Gambaran retina orang sehat dan pasien retinitis pigmentosa (Hartong et al., 2006)
The management or treatment for patients with retinitis pigmentosa currently focuses on maintaining the quality of life for sufferers and slowing down the retinal damage process. This is achieved by providing visual aids such as glasses, advising against smoking, consuming foods rich in lutein and vitamin A, and avoiding direct sunlight exposure to the eyes (Smith et al., 2012). The administration of vitamin A and lutein supplements is believed to prevent degeneration in the retina due to their antioxidant effects. However, the effectiveness of these supplement therapies in clinically improving patients with retinal degenerative diseases, including retinitis pigmentosa, is still debated.
In recent developments, studies on the use of mesenkimal alogenik stem cells have begun. One alternative for allogeneic stem cells is mesenchymal stem cells derived from umbilical cord tissue. The advantages of these umbilical cord mesenchymal stem cells include a non-invasive tissue collection process, high self-renewal capacity, significant paracrine and immunomodulatory abilities. Additionally, umbilical cord mesenchymal stem cells show low human leukocyte antigen-II (HLA-II) and molecule major histocompatibility complex (MHC) class I molecules, minimizing the risk of rejection during transplantation (Sriramulu et al., 2018) Umbilical cord mesenchymal stem cells can regulate inflammatory responses and release biomolecules from paracrine signals that can stimulate pigment epithelium in the retina or produce tropic factors similar to those released by the retinal Pigment eEpithelium (RPE) (Maxson et al., 2012; Han et al.,2020; Ozmert & Arslan, 2020).
ProSTEM collaborates with Dr. Muhammad Bayu Sasongko, Sp.M, M.Epid, PhD at Dr. Sardjito Hospital in Yogyakarta and Dr. dr. Cosmos O. Mangunsong, SpM(K) at Jakarta Eye Center Hospital to conduct clinical trials to assess the potential of stem cells for treating Retinitis Pigmentosa.
Reference
1 Hamel Christian. (2006). Review: Retinitis Pigmentosa. Orphanet Journal of Rare Disease. (40)12.
2 Han, D., Zheng, X., Wang, X., Jin, T., Cui, L. and Chen, Z. (2020). Mesenchymal Stem/Stromal Cell-Mediated Mitochondrial Transfer and the Therapeutic Potential in Treatment of Neurological Diseases. Stem Cells International. doi: 10.1155/2020/8838046.
3 Hartong, D.T., Berson, E.L., and Dryja, T.P. (2006). Retinitis pigmentosa. Lancet, 368: 1795–809.
4 Özmert, E. and Arslan, U. (2020). Management of retinitis pigmentosa by Wharton’s jelly derived mesenchymal stem cells: preliminary clinical results. Stem Cell Research & Therapy, 6, pp. 1–16.
5 Smith Henry B, Chandra Aman, Zambarakji Hadi. (2012). Grading Severity in Retinitis Pigmentosa Using Clinical Assesment, Visual Acuity, Perimetry, and Optical Coherence Tomgraphy. International Ophtalmology. 23-30.
6 Sriramulu, S., Banerjee, A., Di Liddo, R., Jothimani, G., Gopinath, M., Murugesan, R., Pathak, S. (2018). Concise Review on Clinical Applications of Conditioned Medium Derived from Human Umbilical Cord-Mesenchymal Stem Cells (UC-MSCs). International journal of hematology-oncology and stem cell research, 12(3), 230–234.


