These degenerative diseases include diabetes mellitus 1, SLE, multiple sclerosis, arthritis reumatoid, and others. 2 The use of UC-MSCs can help prevent degenerative diseases by their ability to regenerate damaged cells in the body.
Umbilical Cord Blood (UCB), also commonly known as Cord Blood, is the blood found in the baby's umbilical cord and can be collected right after the baby is born. UCB contains various types of stem cells such as Hematopoietic Stem Cells, Mesenchymal Stromal Cells, Endothelial Progenitor Cells.3 Cord blood stem cell transplantation has been performed since 1988 to treat cases of Fanconi Anemia, which is a blood disorder caused by a genetic abnormality. Because it does not involve invasive procedures, cord blood collection is a simple process that poses no risk to the mother or baby and does not violate ethical codes. Cord blood can be stored at extreme temperatures <-150° to be used later without losing its function and quality4 Since then, stem cell therapy (SCT) has rapidly advanced and is widely applied to treat various diseases, such as Acute Lymphoblastic Leukemia (ALL), Acute Myeloid Leukemia (AML), Non-Hodgkin Lymphoma (NHL), Thalassemia, and various other diseases.5 Stem cells derived from UCB (Umbilical Cord Blood) are typically stored and used for autologous therapy, which means stem cell therapy from and for the patient themselves. However, it's also possible for these stem cells to be used for other family members (Allogenic) from the same donor, provided that compatibility requirements between the donor and recipient are met.6 Stem cell storage, whether for autologous or allogeneic therapy, can be done at UCB Bank. ProSTEM is one of the private cord blood banks, so the storage is exclusively for private clients.
Advantages of UC & UCB Storage in ProSTEM
1. ProSTEM has official permission as a cord blood bank and adheres to national and international standards for the management and storage of stem cells.
ProSTEM, as one of the private umbilical cord blood banks in Indonesia, has obtained official permission for the management and storage of stem cells, namely based on the Regulation of the Ministry of Health of the Republic of Indonesia No. 48 Year 2012 concerning the Operation of Umbilical Cord Blood Stem Cell Banks. The management and storage standards of stem cells at ProSTEM also adhere to international standards established by Association of Blood Banks (AABB) and Foundation for the Accreditation of Cellular Therapy-NetCord (FACT-NetCord).
2. Complete facilities for storage and processing of stem cells
The ProSTEM laboratory and stem cell processing and storage facilities are equipped with cutting-edge technologies to support the safe and high-quality processing and storage of stem cells.
3. Monitoring the facilities and the quality of stem cells periodically
Furthermore, ProSTEM conducts regular monitoring of the quality of stored stem cells to ensure that they remain safe and maintain their quality during storage. Monitoring of the storage facility temperature is also carried out 24 hours a day. The results of this quality and facility monitoring are routinely communicated to clients, so they can be assured that the stem cells stored at ProSTEM are always guaranteed in terms of both quality and safety.
4. Comprehensive and standardized services
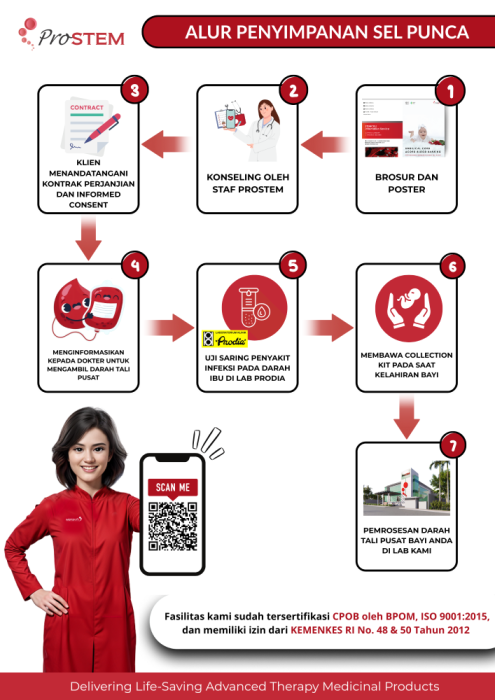
ProSTEM is the only stem cell laboratory offering the most comprehensive services. These include stem cell processing for clinical therapy, stem cell storage from various cell sources, as well as both laboratory-scale and clinical trial research. All services comply with the prevailing standards in Indonesia and even internationally. Some of the advantages of ProSTEM's stem cell storage services are its laboratory facilities certified under Good Manufacturing Practice (CPOB) by the Indonesian National Agency of Drug and Food Control (Badan POM RI), the infectious disease testing process for mothers' blood at Prodia clinical laboratory which is also certified, and the storage of mothers' blood serum and baby plasma at -80° with 24-hour CCTV security and fingerprint access, ensures that the storage security is always maintained.
References
- Stefanska K, Bryl R, Hutchings G, Shibli JA, Konwinska MD. Human umbilical cord stem cells – the discovery, history and possible application. 2020. Medical Journal of Cell Biology; DOI: 10.2478/acb-2020-0009.
- Miryam Mebarki, Abadie, C., Jérôme Larghero, & Cras, A. (2021). Human umbilical cord-derived mesenchymal stem/stromal cells: a promising candidate for the development of advanced therapy medicinal products. 2021. Stem Cell Research & Therapy, 12(1). DOI: 10.1186/s13287-021-02222-y
- Xi, Y., Yue, G., Gao, S., Ju, R., & Wang, Y. Human umbilical cord blood mononuclear cells transplantation for perinatal brain injury. 2022. Stem Cell Research & Therapy, 13(1). DOI: 10.1186/s13287-022-03153-y
- Um, S., Ha, J., Soo Jin Choi, Oh, W., & Hye Jin Jin. (2020). Prospects for the therapeutic development of umbilical cord blood-derived mesenchymal stem cells. 2020. World Journal of Stem Cells, 12(12), 1511–1528. DOI: 10.4252/wjsc.v12.i12.1511
- Gupta AO and Wagner JE (2020) Umbilical Cord Blood Transplants: Current Status and Evolving Therapies. 2020. Front. Pediatr. 8:570282. DOI: 10.3389/fped.2020.570282.
- Little, A., Arash Akbarzad‐Yousefi, Anand, A., Natalia Diaz Burlinson, Paul, Evseeva, I., Latham, K., Poulton, K., Railton, D., Vivers, S., & Wright, P. A. BSHI guideline: HLA matching and donor selection for haematopoietic progenitor cell transplantation. 2020. International Journal of Immunogenetics, 48(2), 75–109. DOI: 10.1111/iji.12527
Storage Flow of Umbilical Cord Tissue and Umbilical Cord Blood Stem Cells