Cirrhosis is an advanced stage of chronic liver disease characterized by fibrosis and damage to the liver structure. Globally, it is estimated that more than 50 million people worldwide are affected by chronic liver disease, with the three most common causes of cirrhosis being alcohol, non-alcoholic steatohepatitis (NASH), and viral hepatitis (Sarin & Maiwal, 2017). According to the World Health Organization (WHO), approximately 257 million people worldwide are infected with hepatitis B, and 20-30% of those with chronic infection will develop cirrhosis and/or liver cancer (WHO, 2017). To date, management of patients with cirrhosis is limited, and liver transplantation remains the only definitive therapy for both cirrhosis and liver cancer patients. However, this approach faces several challenges, including limited donors, post-operative complications, immunological rejection, and high medical costs (Eom et al., 2015; Shi et al., 2012). Several innovations in therapy are emerging to address these challenges, especially for those who have progressed to decompensated cirrhosis and liver carcinoma.
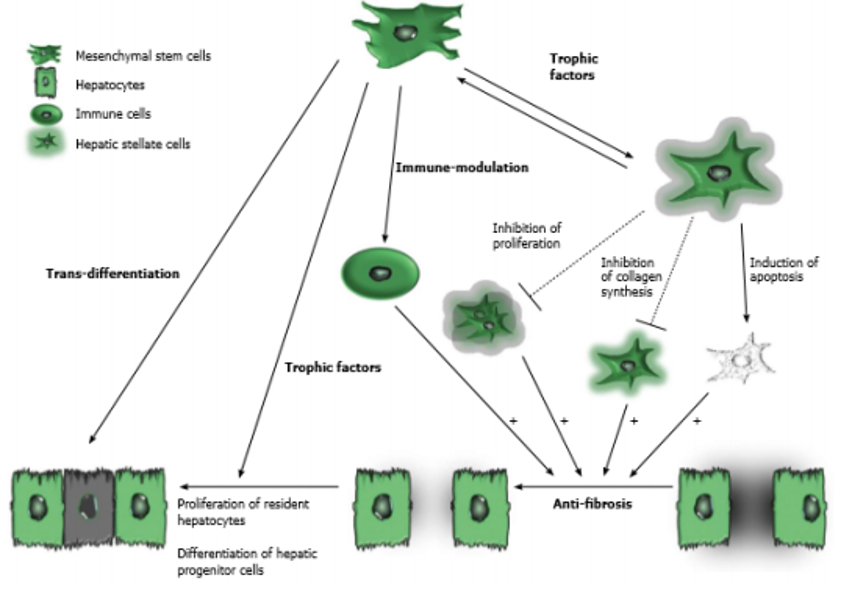
The potential role of mesenchymal stem cells in cirrhosis
(source: Eom et al., 2015)
Cell therapy using mesenchymal stem cellsmesenchymal stem cell Cell therapy using mesenchymal stem cells (MSCs) has been extensively studied as an effort to develop alternative strategies for such issues. MSCs are considered a potential source of cell therapy due to their ability to differentiate into other cell types and exhibit diverse functions, such as anti-fibrosis, enhancing hepatocyte regeneration, improving liver function, and anti-inflammatory properties. MSCs have low immunogenicity and immunomodulatory effects, thus reducing immune rejection. Additionally, MSCs are known to be resistant to reactive oxygen species (ROS) in vitro, reducing oxidative stress in rats, and accelerating hepatocyte growth after liver damage. Human umbilical cord is a promising source of MSCs. This is because the umbilical cord is a 'waste' from childbirth, making the umbilical cord extraction procedure painless and ethical. According to Zhao et al., MSCs from the umbilical cord are superior due to the young tissue age and low infection rate compared to MSCs from adult tissues (Wang et al., 2016).
Currently, ProSTEM, along with the research team led by Dr. Chyntia Olivia MJ, Sp.PD-KGEH, PhD, is conducting clinical trials titled "PHASE I/II ALLOGENEIC UMBILICAL CORD MESENCHYMAL STEM CELL TRANSPLANTATION IN PATIENTS WITH HEPATITIS B-INDUCED CIRRHOSIS" at Cipto Mangunkusumo Hospital, Central Jakarta, with ethical approval number: 0097/UN2.F1/ETIK/2018. It is hoped that this research will enhance services in the field of hepatology, particularly in the area of cirrhosis therapy.
Reference
1 Sarin SK, Maiwall R. Global burden of liver disease: a true burden on health sciences and economies [article on internet]. Cited on October 30, 2017 at 10.14. Available at http://www.worldgastroenterology.org/publications/e-wgn/e-wgn-expert-point-of-view-articlescollection/global-burden-of-liver-disease-a-true-burden-on-health-sciences-and-econom
2 World Health Organization. Hepatitis B [article from internet]. July 2017 [cited on October 30, 2017, at 10.59]. Available from http://www.who.int/mediacentre/factsheets/fs204/en/
3 Eom YW, Kim G, Baik SK. Mesenchymal stem cell therapy for cirrhosis: present and future perspectives. World J Gastroenterol. 2015;21(36): 10253-61. 5. Shi M, Zhang Z, Xu R, Lin H, Fu J et al. Human mesenchymal stem cell transfusion is safe and improves liver function in acute-on-chronic liver failure patients. Stem Cells Transl Med. 2012;1(10): 725-31
4 Wang Y, Yu X, Chen E, Li L. Liver-derived human mesenchymal stem cells: a novel therapeutic source for liver diseases. Stem Cell Research & Therapy. 2016; 7:71.


